The Ubiquitous and Essential: Exploring the Applications of Hydrochloric Acid
Related Articles: The Ubiquitous and Essential: Exploring the Applications of Hydrochloric Acid
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to The Ubiquitous and Essential: Exploring the Applications of Hydrochloric Acid. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Ubiquitous and Essential: Exploring the Applications of Hydrochloric Acid

Hydrochloric acid (HCl), a strong mineral acid, is a ubiquitous chemical found in various natural and industrial settings. Its significance lies in its diverse applications, ranging from industrial processes to biological functions. This article delves into the multifaceted world of hydrochloric acid, exploring its key roles and highlighting its importance in numerous fields.
The Nature of Hydrochloric Acid
Hydrochloric acid, a colorless, highly corrosive liquid, is a strong acid, meaning it readily donates protons (H+) in aqueous solutions. This property underpins its diverse applications, enabling it to participate in a wide range of chemical reactions.
Industrial Applications: A Cornerstone of Chemical Production
Hydrochloric acid plays a crucial role in numerous industrial processes, acting as a vital reagent in the production of various chemicals and materials. Its applications in this realm are extensive and impactful:
-
Production of Inorganic Compounds: Hydrochloric acid serves as a key ingredient in the production of various inorganic compounds, including metal chlorides, such as zinc chloride and iron chloride. These compounds are essential components in various industries, including metallurgy, textiles, and pharmaceuticals.
-
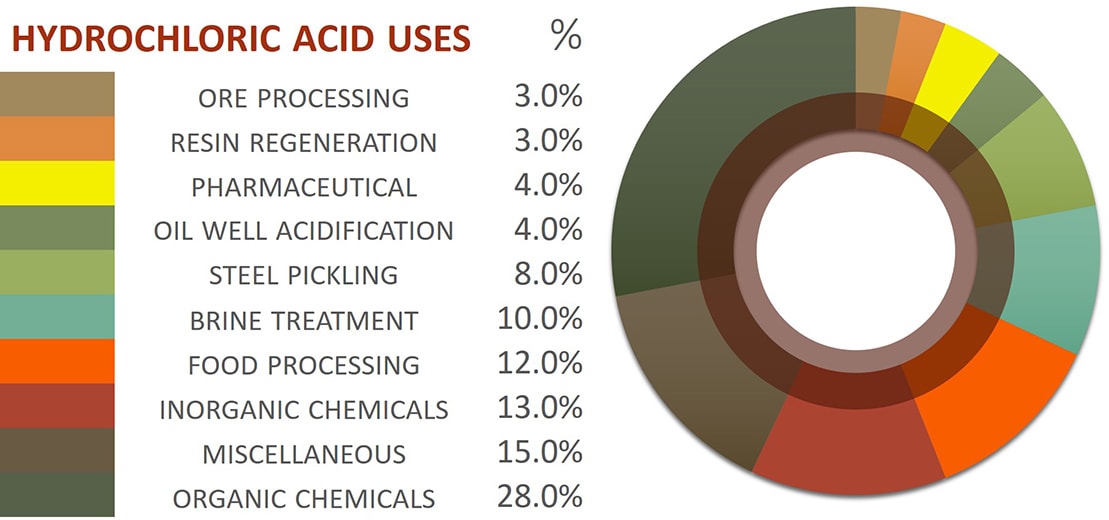
Pickling of Metals: In the metal industry, hydrochloric acid is used for "pickling," a process that removes impurities and oxides from the surface of metals, particularly steel. This process enhances the metal’s corrosion resistance and prepares it for further processing, ensuring the production of high-quality metal products.
-
Leather Tanning: Hydrochloric acid plays a critical role in the tanning of leather, a process that transforms animal hides into durable and usable leather. It aids in removing hair and other impurities from the hides, preparing them for the tanning process, which imparts strength and flexibility to the leather.
-
Food Processing: While seemingly unexpected, hydrochloric acid finds its way into the food industry. It is used in the production of certain food additives, such as citric acid, and in the processing of sugar and other food products.
-
Wastewater Treatment: Hydrochloric acid is employed in wastewater treatment to neutralize alkaline waste streams, effectively regulating the pH of wastewater, which is essential for environmental protection.
Biological Significance: A Vital Component of the Digestive System
Hydrochloric acid is not only a key player in industrial processes but also plays a crucial role in the human body. It is a vital component of gastric juice, secreted by the stomach’s lining. This acidic environment serves several critical functions:
-
Digestion: Hydrochloric acid in the stomach helps break down food, particularly proteins, into smaller molecules that can be absorbed by the body. This process is essential for the efficient extraction of nutrients from food.
-
Killing Pathogens: The highly acidic environment of the stomach acts as a natural barrier against ingested pathogens, killing bacteria and other harmful microorganisms that may be present in food. This protective mechanism is essential for maintaining gut health.
-
Activation of Enzymes: Hydrochloric acid activates certain digestive enzymes, such as pepsin, which are responsible for breaking down proteins. This activation process ensures efficient digestion and nutrient absorption.
Examples of Hydrochloric Acid in Action
To further illustrate the diverse applications of hydrochloric acid, let’s delve into specific examples:
-
Hydrochloric Acid in Pharmaceuticals: In the pharmaceutical industry, hydrochloric acid is used in the production of various drugs, including antibiotics, antihistamines, and analgesics. It plays a crucial role in the synthesis of active pharmaceutical ingredients, ensuring the efficacy and safety of these medications.
-
Hydrochloric Acid in Battery Production: Hydrochloric acid finds application in the production of batteries, particularly lead-acid batteries, a common type used in vehicles. It is used to activate the lead plates in the battery, facilitating the electrochemical reactions that generate power.
-
Hydrochloric Acid in the Production of Plastics: Hydrochloric acid is involved in the production of various plastics, including polyvinyl chloride (PVC), a widely used material in construction, packaging, and other industries. It plays a role in the polymerization process, forming the long chains of molecules that constitute plastic.
-
Hydrochloric Acid in the Production of Dyes: Hydrochloric acid is used in the production of various dyes, including those used in textiles, paints, and inks. It acts as a catalyst in certain dye synthesis reactions, facilitating the formation of the desired color molecules.
Frequently Asked Questions about Hydrochloric Acid
Q: Is hydrochloric acid safe to handle?
A: Hydrochloric acid is a corrosive and hazardous substance. It can cause severe burns on contact with skin, eyes, or mucous membranes. Proper safety precautions, including protective clothing, gloves, and eye protection, are essential when handling this acid.
Q: How is hydrochloric acid produced?
A: Hydrochloric acid is primarily produced through the reaction of chlorine gas with hydrogen gas. This process is highly exothermic and requires careful control to ensure safety.
Q: What are the environmental concerns associated with hydrochloric acid?
A: While hydrochloric acid is essential for various industries, its improper handling and disposal can pose environmental risks. Acid spills can contaminate water sources, harming aquatic life. Proper storage, transportation, and waste management are crucial to minimize these risks.
Tips for Safe Handling of Hydrochloric Acid
- Always wear appropriate personal protective equipment, including gloves, goggles, and a lab coat.
- Handle hydrochloric acid in a well-ventilated area, as its fumes can be irritating.
- Avoid contact with skin, eyes, and mucous membranes.
- Store hydrochloric acid in a secure and well-labeled container, away from incompatible substances.
- In case of accidental contact, immediately flush the affected area with copious amounts of water and seek medical attention.
Conclusion: A Powerful Chemical with Diverse Applications
Hydrochloric acid, a powerful and versatile chemical, plays a vital role in a wide range of industries and biological processes. From industrial production to human digestion, its applications are multifaceted and essential for modern society. Understanding the properties and applications of hydrochloric acid is crucial for its safe and responsible use, ensuring its continued contribution to various fields while minimizing potential environmental risks.






Closure
Thus, we hope this article has provided valuable insights into The Ubiquitous and Essential: Exploring the Applications of Hydrochloric Acid. We hope you find this article informative and beneficial. See you in our next article!