The Chemistry of Clean: Understanding High pH Substances in Your Home
Related Articles: The Chemistry of Clean: Understanding High pH Substances in Your Home
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to The Chemistry of Clean: Understanding High pH Substances in Your Home. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Chemistry of Clean: Understanding High pH Substances in Your Home
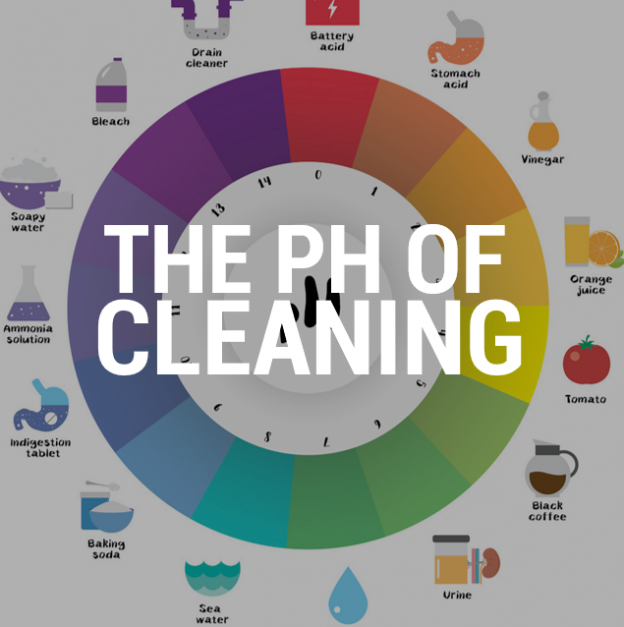
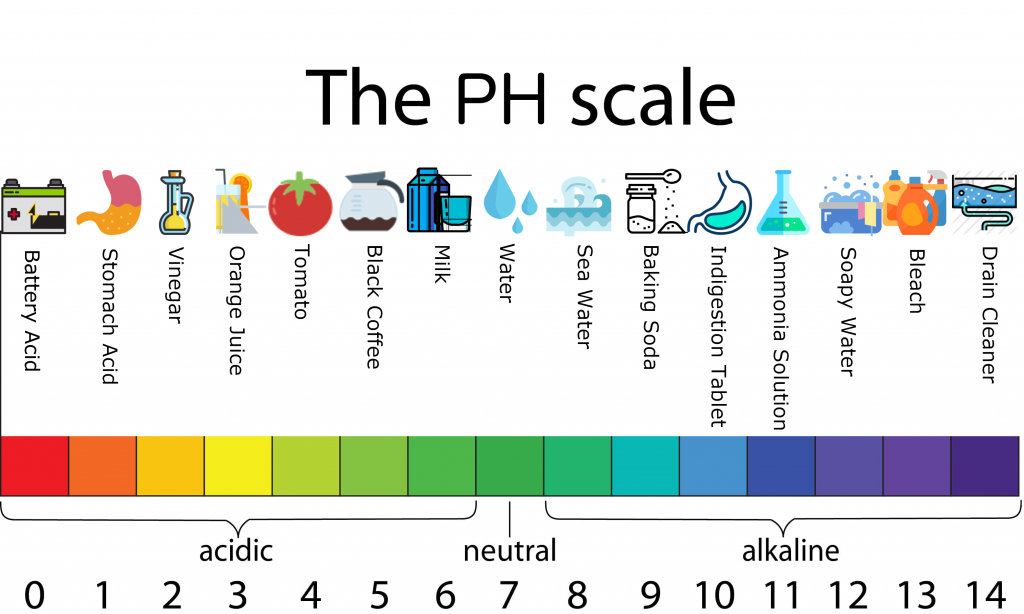
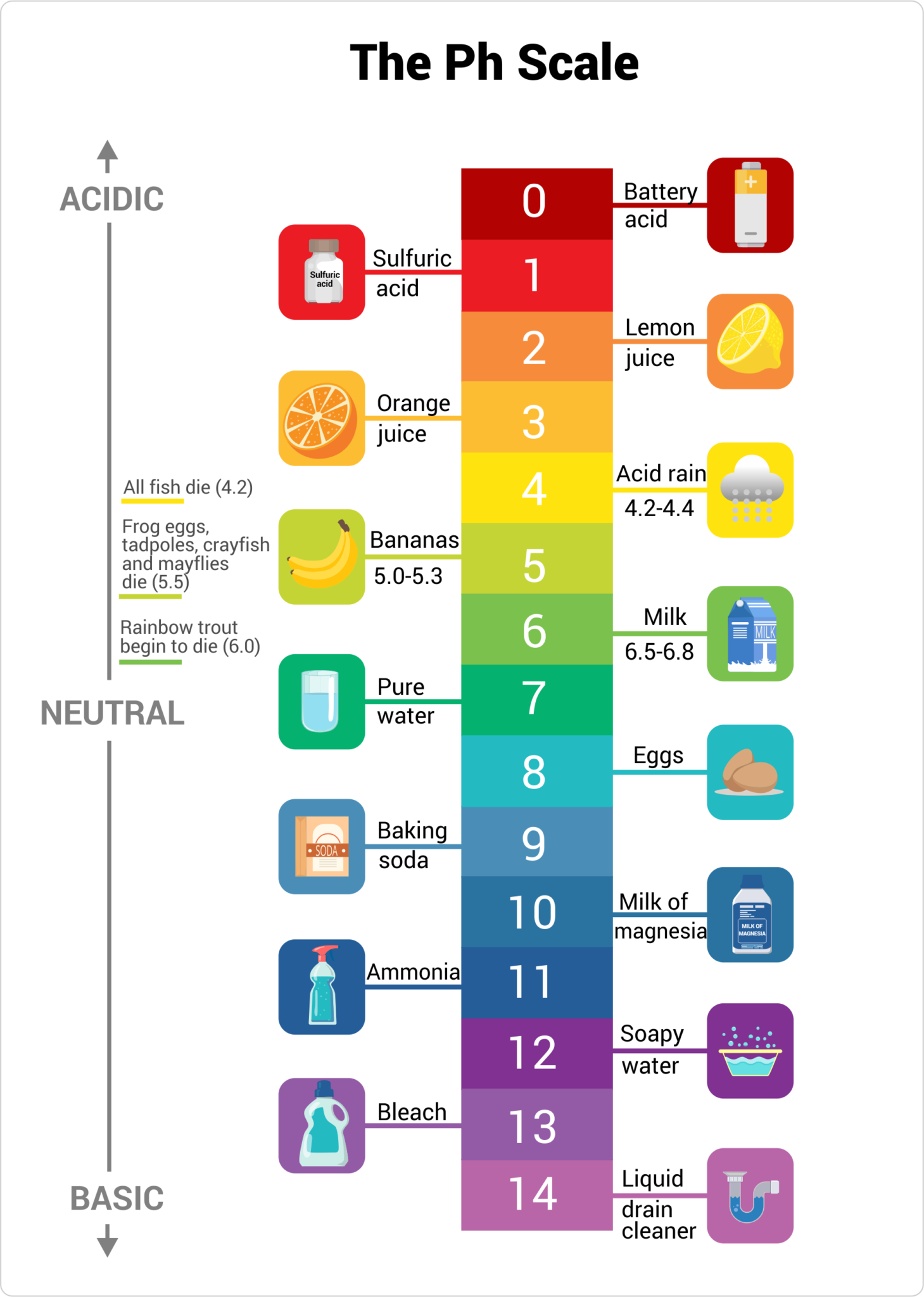
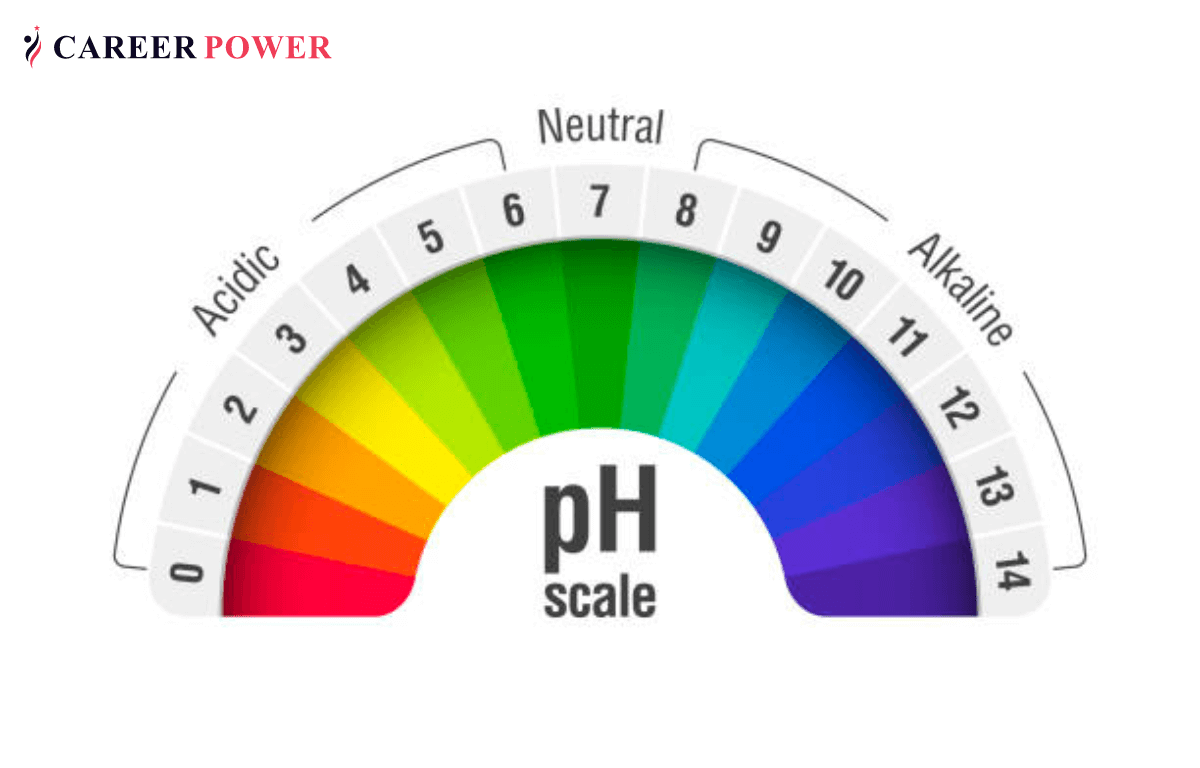
The pH scale, ranging from 0 to 14, measures the acidity or alkalinity of a solution. Substances with a pH above 7 are considered alkaline, or basic, while those below 7 are acidic. While we often associate acidity with things like lemon juice and vinegar, there are many everyday household items that exhibit alkaline properties. These substances, often referred to as "high pH" products, play a significant role in our daily lives, contributing to both cleanliness and safety.
The Power of Alkalinity:
Alkaline substances are known for their ability to neutralize acids. This property is particularly useful in cleaning and sanitation. Many household cleaners utilize alkaline ingredients to break down grease, grime, and other organic matter. The high pH disrupts the chemical bonds holding these substances together, effectively loosening them for removal.
Common High pH Household Items:
- Cleaning Products: The most common examples of high pH substances in the home are cleaning products. Dishwashing detergents, oven cleaners, drain cleaners, and many all-purpose cleaners often contain alkaline ingredients like sodium hydroxide (lye) or potassium hydroxide. These strong alkalis effectively break down grease, food residues, and other stubborn stains.
- Soaps and Detergents: While not as alkaline as strong cleaners, soaps and detergents also exhibit a higher pH than neutral water. This alkalinity helps lift dirt and grime from fabrics and surfaces, making them effective for laundry and dishwashing.
- Baking Soda: A familiar pantry staple, baking soda (sodium bicarbonate) has a pH of around 8.3. This mild alkalinity makes it a versatile cleaning agent, suitable for deodorizing, scrubbing, and removing mild stains.
- Ammonia: Another common household cleaner, ammonia has a pH of around 11.5. It is a powerful degreaser and disinfectant, often used in window cleaners and floor polishes.
- Borax: Borax (sodium borate) is a naturally occurring mineral with a pH of around 9.5. It is a popular ingredient in laundry detergents and stain removers, known for its cleaning and deodorizing properties.
- Lime: Calcium hydroxide, commonly known as lime, is a strong alkali with a pH of around 12.4. It is used in construction and agriculture, but can also be found in some cleaning products.
Benefits and Considerations:
High pH substances bring numerous benefits to our daily lives. Their cleaning power makes them indispensable for maintaining hygiene and sanitation. However, it is essential to use these products responsibly and with caution.
- Safety First: High pH substances can be corrosive and irritating to skin, eyes, and respiratory systems. Always wear protective gear like gloves and eye protection when handling these products.
- Dilution and Storage: Always follow the manufacturer’s instructions for dilution and storage. Using too concentrated a solution can lead to damage and potential harm.
- Mixing Precautions: Never mix different cleaning products, especially those containing ammonia and bleach. Mixing these substances can create toxic fumes.
- Environmental Impact: Some alkaline cleaning products contain chemicals that can be harmful to the environment. Choose eco-friendly options whenever possible.
FAQs about High pH Substances in the Household:
1. What is the difference between acidic and alkaline cleaners?
Acidic cleaners are typically used for removing mineral deposits, rust, and lime scale. They are effective for cleaning surfaces like toilets and bathroom fixtures. Alkaline cleaners, on the other hand, are more effective against organic matter like grease, grime, and food residues.
2. How can I neutralize a spilled alkaline solution?
In case of a spill, use a mild acid like vinegar to neutralize the alkaline solution. Always wear protective gear and avoid mixing with other chemicals.
3. What are the signs of a high pH substance?
High pH substances often have a slippery feel and can cause a burning sensation if they come into contact with skin or eyes. They may also have a strong odor.
4. How can I safely dispose of high pH cleaners?
Always follow the manufacturer’s instructions for disposal. Never pour these substances down the drain unless it is specifically recommended. Contact your local waste management authority for proper disposal guidelines.
Tips for Using High pH Substances Safely:
- Read the label: Always read the product label carefully before using any cleaning product.
- Ventilate the area: Use high pH cleaners in well-ventilated areas to avoid inhaling fumes.
- Wear protective gear: Always wear gloves, eye protection, and appropriate clothing when handling these products.
- Store safely: Store high pH cleaners in their original containers, out of reach of children and pets.
- Avoid mixing: Never mix different cleaning products, especially those containing ammonia and bleach.
Conclusion:
High pH substances play a crucial role in maintaining a clean and healthy home. Understanding their properties and using them safely is essential for both personal well-being and environmental protection. By following the tips and guidelines outlined above, you can effectively utilize the power of alkalinity while minimizing any potential risks.




Closure
Thus, we hope this article has provided valuable insights into The Chemistry of Clean: Understanding High pH Substances in Your Home. We appreciate your attention to our article. See you in our next article!